Search the Community
Showing results for tags 'tds'.
-
Greetings shrimp enthusiasts, hobbyists, keepers and those with curiousity and a passing interest, I was once one with a passing interest that'd grown into a curiousity and now i'm heading for the keeper stage -- hopefully a keeper of Riffle Shrimp. Below is my 36" x 18" x 18" tank. As you can see it's a planted tank. It completed its Nitrogen Cycle today -- it's a happy day that's been a long time in the making. We purchased the tank in Feb of 2015 and what with moving house soon after and our 3rd baby it's taken this long to get to this exciting day. I've had tanks with shrimps before with what I'd describe as limited success, but it's been many years and with the knowledge, equipment and technology that's around today I feel like I've just begun as a new aquarist all over again. As yet we have no fish, let alone shrimp, but that is likely to change in the next few days. We intend the tank to be a community one but with a key feature being some shrimp. I saw some Riffles in a display tank in a fish store and was immediately drawn to them well ahead of the various smaller shrimps that they had for sale. They didn't and still don't have any Riffles for sale. I trust that I will one day get some, somehow, so I am aiming to prepare my tank for that time and thought it might be good to ask the SKF Aquatics community for guidance and advice -- not just be a fly on the wall reading other's posts. Tank specs: 36 x 18 x 18 inches (approx 90 x 45 x 45 cm); about 50 gal (190 litres) Substrate: ADA Malaya (not a super nutrient rich variety as we plan to have a low tech tank in the long run) DW: Golden Vine Rocks: Red rock Plants: Monte Carlo (Low-tech thriving), three varieties of Crypts, Amazon Sword, Lileopolis, Rotala Walichii, tiny bit of Val, tiny bit of Telanthera, Anubis mini nana Fluval 406 Cannister Filter (pretty hi-flow/circulation is how i understand it for my tank size) Hydor inline heater UP Aqua U Series P LED light Hydor Koralia Wavemaker (not used at present) Fish/Invertebrates we have in mind (but are by no means fixed choices): 5 x White Fin Ornate Tetra (a docile, hardy tetra that is highly recommended as a fish for a freshly cycled tank) that aren't schooling, but more shoaling) 3 x Otocinclus (for algae should it one day inevitably strike) 1-2 x Chain Loaches (aka dwarf loach) (to deal with some pond snails and is hopefully compatible with Riffles) 20-ish x Cardinal Tetras (hardy and schooling) 5-7 x Celestial Pearl Danio 1-2 x Borneo Catfish Pair x Blue Rams Colony of Riffles Is the above list suitable tank-mates with Riffles? All are pretty docile as I understand it. As I said before, the tank just completed cycling. I plan to test its stability, but using ADA Aquasoils they are said to be able to handle a high bio-load once cycled due to their nature to leech a lot of ammonia. If the cycle is stable then it's possible it's ready to load the tank up with quite a bit. January 10 I began the hardscape and the next day planted as much as I could envisage would fit once the plants grow out. We have an 8 hour photoperiod and plants have been growing very well without CO2 or ferts. We have had ZERO algae outbreak (touch wood) aside from a tiny tiny bit of brown algae on the DW along with protein slime, which the pond snail hatch-lings cleaned, feasted, thrived on, and even laid eggs upon already. Water testing 2-3 times a day I have watched and kept a detailed journal of the cycling process. Today, as planned for the day the cycle completed, I dosed the tank for the first time with Seachem Flourish Excel, some Fluval plant food I had from a few years back, and Seachem Equilibrium. The water we have here in Melbourne is quite soft and a TDS meter reading shows that some added salts and minerals were required (I understand that an ideal aim is for TDS reading of 250, but I'm usually under that). I've added coral bone to the cannister filter as PH (ADA Aquasoils cause PH to drop for a while also) needed buffering up to 7.0 neutral. I'll continue to test the water daily to see that stability is maintained. Water temperature has been high at 29 Degrees C to speed up the Nitrogen Cycle process, as has surface agitation, and these I will adjust these lower once I've confirmed that cycle stability. I need to purchase a KH/GH test kit, but understand it also has a relationship with TDS (hence the aim of TDS 250), but please correct me if I'm mistaken? So aside from a critique of what I have done above I now ask for your learned advice on what I need to do next in preparation for shrimp? I feel I need to let introduce the fish first and get them comfortable, meanwhile it's reasonable to expect that the plants will have accelerated growth with the added carbon from the Flourish. Am I right to understand that Riffle Shrimp would like a jungle-ish environment? I can envisage them sitting on the left hand side of the drift wood where the out flow is highest (the wavemaker also points at that spot and I'd probably set that on a timer to come on several times a day if it's needed) filter feeding. Once I feel I have the right environment and care knowledge I hope I will be able to obtain some Riffle Shrimps with some help from SKF! Then perhaps I can truly move from being just curious through to being an enthusiast with some experience to share/help others. Thank you for reading, hope it's interesting and there aren't too many who look and go TL;DR. Please be gentle: Although I read A LOT of forums, this is the first time I'm posting in any forum in a very long time after finding the experience with keyboard warriors too taxing. I wouldn't normally have taken the time to ask for help in any forum; but, the enthusiasm for the idea of having these captivating shrimp in my planted community tank has surpassed my shyness and found me willing to put myself out there. D
- 15 replies
-
- riffle shimp
- ada malaya
- (and 7 more)
-
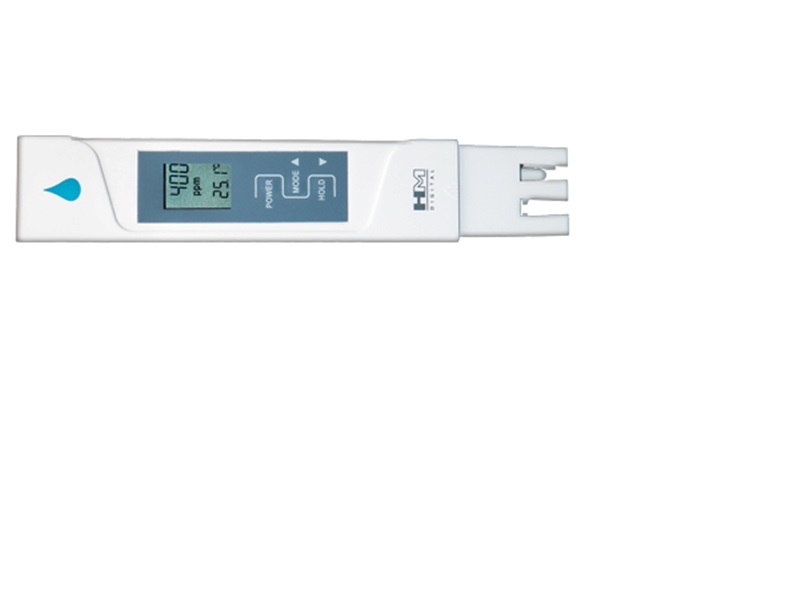
TDS and why is it important Preamble: Total Dissolved Solids or TDS for short is an area of water parameter we talk about very often, and is usually one of the first things we ask about when checking water parameters. This article will hopefully go into some depth for anyone who might still be new to the hobby, and likewise, might teach the veterans a thing or two that they might not have known about TDS. I have tried to keep the language appropriate to newcomers in mind, so please don’t expect a paper that reads like a scientific thesis. This article is also written from the perspective of a fish and a shrimp keeper, as I am, and draws from my experiences in these areas. You will see frequent mention of killis, Apistos, and shrimps. For the sake of simplicity, we will regard Electrical Conductivity (EC) to be of similar importance and similar definition to TDS. A definition of EC is the measure of the water's ability to "carry" an electrical current and indirectly, a measure of dissolved solids or ions in the water. Whereas a definition of “Total Dissolved Solids (TDS) is the total amount of mobile charged ions, including minerals, salts or metals dissolved in a given volume of water, expressed in units of mg per unit volume of water (mg/L), also referred to as parts per million (ppm). TDS is directly related to the purity of water and the quality of water purification systems and affects everything that consumes, lives in, or uses water, whether organic or inorganic, whether for better or for worse.” – (source: HM Digital) From the perspective of an aquarist, TDS can be defined as: a count of all the dissolved inorganic solids in the water. TDS gives an overview of mineral content in the water. It does not just necessarily provide information on hardness even though it does include the measurement of minerals like calcium and magnesium. Instead, TDS also includes measurements of all the other dissolved minerals in the body of water. So you cannot use TDS to give you an indicator of hardness, that is, how much calcium carbonate is dissolved in the water. GH is at its heart a measure of divalent cations, namely Ca (calcium) and Mg (magnesium); and we know KH is a measure of carbonate concentration. Both GH and KH can influence hardness and TDS levels – ‘an aquarium high in GH & KH can have a high TDS’. However, a fish tank could have a high TDS reading but still have low GH and KH readings. In this situation the aquarium water might be high in one or more of the other dissolved minerals apart from Calcium and Magnesium. Therefore, TDS is a better reflection of the total mineral content than hardness measurements. In conclusion, Total Dissolved Solids consists of dissolved ionic elements, both cations and anions. Whereas, GH only measures two elements, Calcium and Magnesium. Let’s see what those other minerals, that a TDS meter/pen measures, might be. In chemical terms, if a neutral atom loses one or more electrons, it has a net positive charge and is known as a cation (source: Wikipedia). Cations are elements that can be found mainly on the left side of the periodic table (metals) and when it reacts, they usually become positive ions. Cations include ions such as calcium, magnesium, potassium, sodium, barium, iron, copper and zinc. If an atom gains electrons, it has a net negative charge and is known as an anion (source: Wikipedia). Anion elements can be found on the right side of the periodic table which reacts with metals to take electrons to form negative ions called anions. Anions include elements such as chloride, nitrate, iodine, bromide, fluoride, sulphide, chlorate, permanganate, phosphate and sulphate. Because of their electric charges, cations and anions attract each other and readily form ionic compounds, such as salts. All these ions and other inorganic ions are what is included in the measurement of TDS. Occasionally you will also hear of the term Total Suspended Solids (TSS). Therefore Total Suspended Solids refers to solids both suspended and dissolved in water and is directly related to conductance and turbidity (optical determination of water clarity – how cloudy/clear the water is). Dissolved solids (invisible) are therefore the substances that can flow through the filter media, too small to be trapped. And the substances in TSS include undissolved solids (visible), like bits of plant matter, or detritus and therefore includes substances that can be trapped by the filter media. High levels of TSS also have the following impacts: increased levels of TSS obstruct light and therefore reduce photosynthetic absorption in plants. High TSS can gradually decrease the amount of oxygen produced by these plants. Decaying plant matter uses up a lot more oxygen and subsequently reduces the amount of dissolved oxygen available in the water. Unless there is a significant amount of surface agitation (oxygenation). It’s always a good idea to have your filters, be it air driven or canister, break the surface of the water. It will reduce protein scum off the surface and maximise the oxygen exchange. While TSS is not specifically measured in a TDS meter, it’s good to know the difference between TDS and TSS, as well as its influence in the aquarium environment. Measuring Total Dissolved Solids TDS is the measuring of the amount of salts in a solution. For a lot of applications the amount of salt is indicative of the levels of other stuff in a solution. TDS/PPM meters sold for gardening and aquariums figure the amount of salt in Parts Per Million by measuring the Electrical Conductivity of the solution under test. So a PPM/TDS meter is an EC meter that converts the EC value into PPM values. EC is a measure of Electrical Conductivity from two probes 1cm apart. 1 EC is = 1 microsiemens, to convert from EC to siemens multiply by 1E-6. EC can be converted to PPM by multiplying by 500. PPM can be converted to EC by dividing by 500. To convert from siemens to Ohms is s=1/ohms, you can also go the other way and do ohm=1/s for siemens to ohms. Siemens is also known as Mhos, which comes from ohm written backward. The number 500 used to convert between PPM and EC is called the Conversion Factor. Different salts will have different conversion factors because some conduct better or worse than others. NaCl's is 500, this seems to be the most common standard used, and is what was used for the calibration solutions. Though there is a close relationship between TDS and Electrical Conductivity, they are not the same thing. Total Dissolved Solids (TDS) and Electrical Conductivity (EC) are two separate parameters. TDS, in layman's terms, is the combined total of solids dissolved in water. EC is the ability of something to conduct electricity (in this case, water's ability to conduct electricity). The measurement of dissolved solids is expressed in ppm of NaCl (sodium chloride) – TDS can be compared to Electrical Conductivity (EC) and the approximate conversion formula to get TDS(ppm) = 0.64 x EC mS/cm Conductivity measures electrolytes. Aquarists can now measure TDS levels via tests performed using a TDS meter (or TDS pen) in ppm at a relatively cheap price. Alternatively, you could use an EC pen and convert to TDS using the manufacturer’s conversion factor. Picture of a TDS meter or TDS pen TDS meters are usually calibrated using a solution of Sodium chloride NaCl. While Electrical Conductivity meters (EC) are usually calibrated with a solution of Potassium Chloride KCl. How do TDS pens work? Two electrodes with an applied AC voltage are placed in the solution. This creates a current dependent upon the conductive nature of the solution. The meter reads this current and displays in either conductivity (EC) or ppm (TDS). Electronic TDS meters essentially measures the conductivity of water, ie. how well the water conducts electricity. The higher the concentration of ions, results in the higher the conductivity of the water, and thus the higher the TDS level will be. And most of our softwater shrimp and fish don’t like high TDS. Many brands have meters that use a conversion ratio to change EC (conductivity in microsiemens) into TDS (ppm) along with a temperature compensation. It really does not matter too much, which one you choose to use, since they use the standard conversion for tap water of 0.5. For example, an EC measurement of 300 mS is converted to a TDS measurement of 150 ppm (TDS = EC x 0.5). In fact, most (if not all) TDS pens are actually EC meters that convert to TDS automatically saving the user from performing the mathematical step. There are TDS meters that perform a combination of functions (TDS/EC/pH/temp) which allow conversions to be adjusted between 0.47 and 0.85. There is one weakness with TDS measurements however, it does not measure which ions are responsible for the conductivity. So if you are testing tap water you don’t know if it’s the “good” ions like Calcium, Magnesium, Potassium or the undesirable ions like Iron, Copper, Nitrates, or any other number of dissolved solids that makes up the abundance of the reading. That is why many experienced aquarist will recommend using RO water and remineralising it so you know exactly what is in the water. A few general observations on TDS When water reaches a TDS count of 50ppm it becomes electrically conducting, that is, it’s able to conduct electricity at this level. The EPA Secondary Regulations advise a maximum contamination level (MCL) of 500mg/litre (500 parts per million (ppm)) for TDS. Numerous water supplies exceed this level. When TDS levels exceed 1000mg/L it is generally considered unfit for human consumption. A high level of TDS is an indicator of potential concerns, and warrants further investigation. Most often, high levels of TDS are caused by the presence of potassium, chlorides and sodium. These ions have little or no short-term effects, but toxic ions (lead arsenic, cadmium, nitrate and others) may also be dissolved in the water. Higher levels can be a likely cause for corrosion in plumbing. The visual quality of water is also impacted at levels higher than this. A general observation of high TDS water is that it is slightly alkaline in pH, that is it is lacking in hydrogen molecules. As with everything in nature that tries to reach equilibrium, high TDS or alkaline water will want to seek out hydrogen molecules to reach a neutral state. As such, slightly alkaline water often causes dehydration at a cellular level. Low TDS water on the other hand is loaded with positively charged hydrogen molecules and is therefore slightly acidic in pH. Low TDS water is therefore very hydrating at a cellular level. TDS and Low pH fish When we discuss soft water fish or shrimp that like low pH, what that generally means is that these fish are really low TDS fish. While it is not impossible for many of these low TDS fish to adapt to harder water, and higher pH (and often relatively higher TDS levels), the problem is, especially for South American soft water fish and Caridina shrimp, that calcium and magnesium rich water makes the egg’s membrane harder, and dramatically reduces the chances of hatching. And in more recent experiences, I’ve had greater success hatching killi fish eggs in lower TDS levels than at higher TDS levels. I have also observed that high TDS levels (due to high levels of chlorides, calcium and magnesium, fluoride, sulphides as found in tap water) is generally the cause. This is where it can be a common mistake for many people, including myself, to try all sorts of methods to lower pH and hardness but give no attention to TDS values. This is where Reverse Osmosis water can help with this predicament. I now use RO water to successfully lower calcium and magnesium levels, as well as TDS. This in turn has an impact on reducing pH as well as KH and GH. The fish seemed to be much more contented using this method of preparing clean, low TDS water. And as a reward, the fish promptly rewarded its keeper with eggs which hatched into fry (apistos, rams, other South American dwarfs and Killies). If you want to also match the tank water with the shrimp’s or fish's natural habitat parameters, keep the TDS levels low. What is low? Soft water is generally considered to be in the range of TDS 70 – 150ppm. My personal observations have shown me that high levels of dissolved mineral content in the water, are the main reasons for the failed breeding of many Killis, Shrimp (Caridina) and South American dwarf cichlids. This is attributed to inappropriate levels of calcium and magnesium, and subsequently carbonates and bicarbonates. Placing the emphasis solely on pH alone does not rectify the issue since it can be said that pH is a symptom of the overall mineral content in the water, rather than the actual root cause. To make matters worse, pH down chemicals only adds to the TDS count, not decrease it. When breeding low pH fish, keep your emphasis on TDS instead of just pH. Some aquarists can often find themselves in a situation with tap water parameters that are no where near their shrimp’s or fish's preferred water conditions. My tap water in Sydney for example, is pH 7.8+, and GH & KH are also somewhat high for breeding caridina shrimp, Killi, or Apisto dwarf cichlids, which means we need to find a way of reducing it. We need to really stop thinking about just the permanent hardness of pH, KH and GH, or even temporary hardness for that matter. Concentrate on reducing calcium and magnesium hardness instead. Mixing the tap water with just plain RO at a ratio of 50:50 can be the simple solution to solving the problem of TDS, pH and Hardness. We want to keep calcium and magnesium hardness in check since this can affect the fertilisation of the egg, as the egg’s membrane can get too hard to a point of making fertilisation extremely hard (pun) and nearly impossible. Sometimes I will mix RO water with peat treated water along with tap water to make water whose parameters closely approximates the needs of the Killi or South American dwarf cichlids. Tap water can be included in order to stabilise KH levels and thus keep the pH from fluctuating. Occasionally, I will add my own remineralising DIY mix to RO water to bring TDS up to a certain specific level on the occasions that adding tap water was undesirable, especially for my shrimp tanks. TDS readings for my Caridina shrimp are around 140-150, with a lower KH value of 0-1 and GH of around 5-7 have been proving to be successful for me. Neocaridina dwarf shrimps are capable of tolerating slightly higher TDS levels of up to 200. They might survive higher, but it would be unethical of me to advise you that it’s okay beyond 200. TDS readings for Killi and South American cichlids of between 70 and 110ppm with a stable KH reading between 3 and 7. New soft water low pH fish and shrimp don’t merely survive in this treated water, but instead will thrive and multiply. You will find quite frequently that you will need to mix your water changes to a much lower TDS value that your target in order to maintain the tank’s overall TDS. This is normal, as the dissolved solids in the tank is continually increasing from various sources, like fish/shrimp waste, minerals introduced in food, water evaporation, or even decomposition of plants and organic matter. You might even get to a point where you need to change 80%+ of your tank water just to reset the TDS values. Don’t forget to re-acclimatise the shrimp back into this new water. TDS: Water Changes Many professional fish breeders practice the following method of TDS monitoring; it is one important parameter used to keep healthy fish. You could also use TDS levels as a means of deciding on the frequency of when it is time for a water change. A rise in TDS levels means it is time for some water to be changed, returning TDS levels to a lower count. Sharply increasing TDS levels can also indicate overfeeding, an over-stocked tank, or too much added minerals or fertilisers. But I would use caution in relying solely on TDS readings for water change indications. This is best reserved for those that are very familiar with their tank and understands what the TDS reading is showing. “pH Shock” - Moving fish from one tank to another For many years as a fish keeper, and now also as a shrimp keeper, I have understood changing the pH on fish or shrimp too quickly is a bad thing. It was only until I was researching the importance of TDS, a revelation has come to mind. TDS levels can represent different states of osmosis. Many aquarist have largely believed fish that succumb to what we call 'pH shock' is caused by the rapid variation in TDS levels. This places osmotic pressure stresses on the fish's osmoregulatory mechanisms which cannot become accustomed fast enough to the changing environment and hence the fish goes into a state of suffocation and in many cases can cause death. Fish have been shown to withstand fairly significant pH shifts when the TDS was low in both waters. It was not 'pH shock' as it is often alleged– that is, where the difference in pH is significant between one tank to another. But it was TDS shock! Maybe it’s because TDS meters are not as readily available, whereas, pH kits can be found in every fish shop. So the misguided recommendation was to test for pH, rather than TDS – who knows. One could declare that TDS measurements help to give an indication of the differences in osmotic levels between the water of one tank and another. In water with less total dissolved minerals compared to the amount of dissolved minerals in the tissue of the shrimp/fish, will cause the shrimp/fish to lose fluid from its cells via its gills (over hydration). In high TDS water, it has the opposite effect, they become dehydrated. Which causes the fish to have difficulty passing toxins out via its kidneys. This is a longer term impact to the fish, and you might not notice any impact immediately. IMHO, TDS meters are often the most under estimated tool that can be used to give a good indication of how successfully a shrimp or fish will adapt to the water in one tank to another. As a second reference, reading one of J. J. Scheel’s articles on dissolved solids also brought me to this realisation of ‘TDS shock’. Between 1959 and 1965 Col. Jorgen J. Scheel of Denmark sent out some letters about the science and systematics of killifish to any hobbyist that was interested. Scheel had what might be considered today some unorthodox opinions regarding water chemistry. He felt differences in salinity, or total dissolves solids mattered much more than pH (which could be safely ignored). Given this observation was made over many decades of working with killifish, it's a pervasive argument. Here are the relevant passages from Rivulins [killifish] of The Old World: Page 25 Page 26 TDS can also significantly impact the osmoregulation of the gills. Low TDS can cause the red blood cells to be depleted of water in fish that might not be acclimatised to the low TDS. While in high TDS, the red blood cells in the gills can be saturated with water causing the red blood cells to expand. Both will cause respiratory problems. As a result, always drip acclimatise new shrimp or fish to your tank prior to introducing them. Use your TDS meter/pen to match TDS values in your tank and the water of the new shrimp/fish. It usually takes doubling the amount of water from the tank to match the TDS in the bag of the new fish/shrimp. More caution needs to be placed on reducing TDS levels, compared to increasing TDS levels, as the former seems to be more lethal. Methods of lowering TDS There are several methods of lowering TDS, however, we will focus on only two methods as the other methods are unsustainable in the long term. These sources of low TDS water will need to be remineralised with Calcium & Magnesium mix in a ratio of 4:1. Remineralising raises the low TDS water back to a more suitable amount specific to the requirements of your fish or shrimp. Do not use low TDS water except to top up water loss due to evaporation. RO (Reverse Osmosis) Water Reverse osmosis works by forcing water under great pressure against a semi-permeable membrane that allows water molecules to pass through while excluding most contaminants. RO is the most thorough method of large-scale water purification available. There are a huge number and variety of RO systems around. Studies have revealed how the concept of osmotic pressure can assist in decontaminating water. With a fine particulate membrane and the act of forcing water through that membrane with sufficient pressure, will produce clean water on the other side of the membrane. The clean water is stored and the filtered waste is either thrown away or used for other non consumption uses like watering plants. RO systems can removed up to 98% of all ionic and organic impurities like pollutants, sediment, bacteria and contaminants. And as a result, TDS levels are drastically reduced. The RO filter membranes do not last forever unfortunately. As the TDS of the output water rises, it is generally an indication that the membranes need to be changed. The frequency of use and the level of TDS of your source (tap) water will determine the frequency of replacing the membrane. Deionisation (DI) In large scale DI systems water is passed between a positive electrode and a negative electrode. Ion selective membranes allow the positive ions to separate from the water toward the negative electrode and the negative ions toward the positive electrode. High purity de-ionized water results. Deionization is an on-demand process supplying purified water when needed. This is important because water at this extreme purity level degrades quickly. The nuclear grade deionization resin or polishing mixed bed resin removes almost all the inorganic contaminants in the water increasing the resistivity of the water to a maximum of 18.2 megohm-cm. However, deionization alone does not remove all types of contaminants like dissolved organic chemicals. Deionization filters are not physical filters with a pore size and cannot remove bacteria or particulates. The water is usually passed through a reverse osmosis unit first to further remove non-ionic organic contaminants. RO vs DI: RO purity is relatively continuous while DI gets progressively worse as the resin nears its regeneration point. DI chemicals are expensive and therefore operating costs are higher than RO per litre of purified water. RO membranes are a physical barrier that remove bacteria, viruses, algae and suspended solids, while DI systems cannot remove these contaminants. DI uses two hazardous chemicals, hydrochloric acid (HCl) and caustic soda (NaOH) for regeneration of the resin beds. These chemicals needs special storage and disposal requirements. As you can see, DI water is also uneconomical for aquatic hobbyists. More portable DI systems nowadays use Ion exchange resins to exchange non desirable cations & anions; and replaces them with hydrogen and hydroxyl, respectively, forming pure water (H20), which is not an ion. One type of resin will remove positive IONS, while another type of resin will remove negative IONS. Cations Anions Removed by Cation Resins Removed by Anion Resins Calcium (Ca++) Chlorides (Cl-) Magnesium (Mg++) Sulfates (SO4=) Iron (Fe+++) Nitrates (NO3=) Manganese (Mn++) Carbonates (CO3=) Sodium (Na+) Silica (SiO2-) Hydrogen (H+) Hydroxyl (OH-) (Table care of Puretecwater) You might come across the term "Mixed bed" or "Dual Bed" system - this is a DI filter with both Cation and Anion resins. RO/DI portable systems Modern portable RO/DI systems solve both the individuall RO and DI shortcomings. These systems combine an RO membrane with DI resins to produce near 0 TDS water. The RO removes the organic waste like bacteria, viruses and algae that the DI cannot. While the DI removes the minerals like Calcium, Magnesium, Chlorides, Sulfates, etc that the RO membrane misses. By combining the two, we get the best of both worlds. Most Reverse Osmosis filters you can buy today, like those sold by FSA, https://www.filtersystemsaustralia.com.au/store/index.php/reverse-osmosis-water-filter/aquarium-systems.html are in fact RO/DI systems. Rain water What can be better than water from mother nature? After all, our river systems are made up of water that falls as rain. So this has to be the best source of water, is it not? In most cases it is. However, many of us live in polluted cities, and we collect and store rain water in manmade receptacles that might add to the contamination of rain water. So some form of caution is necessary. If you are confident that the water is collected off relatively clean, rust free roofs and stored in plastic drums, then rain water is a perfect free source of low TDS water. Rain can be sporadic and unpredictable in some countries, so an RO system as a backup is always a good idea. There are other methods of lowering TDS, as mentioned in the next section, but I will not focus on them as it’s not really a preference. I mention it here only as a last resort. Peat All over the internet and on forums, many can attest to using peat in helping to lower pH, GH, KH, and TDS. This greatly depends on your own water conditions and how much the peat treated water affects TDS. If your tap water is particularly hard, you might need more peat to lower the mentioned parameters compared with someone else’s tap water. It is not uncommon to mix the peat with RO water (and/or maybe some tapwater) in an attempt for one to achieve a stable chemistry that agrees with the shrimp/fish you are keeping. The addition of tannins, phenols, humic acids along with the combination of peat treated water allows you to create water conditions close to your livestock’s natural environment. Peat water (even small additions) is positively regarded by many aquarists, as essential for low TDS fish, especially dwarfs such as Discus, Tetras, Corys, Angels, Rams and Apistos. The problem with recommending peat is finding it in Australia is difficult. Especially peat that does not also have fertilisers included. Then there is the extra effort in making peat water, and the need to make it several days ahead of use and store it in containers. The colour that results from the added tannins from peat is also not to everyone’s liking. Distillation Distillation involves boiling the water to produce water vapour. The water vapour then rises to a cooled surface where it can condense back into a liquid and be collected. Because the dissolved solids are not normally vaporized, they remain in the boiling solution. However, some impurities with the same boiling point of that of water can be transferred to the collection water, and for this reason, Reverse Osmosis can produce purer water. The absolute advantage of the distilled water is the complete absence of harmful substances like bacteria, viruses or algae. Considerable amount of cost is required to produce and maintain the thermal requirements for a distillation process. As a result this method is uneconomical for aquatic hobbyists. A quick word on Water softeners Water softeners do not necessarily produce water that is suitable for Softwater fish and shrimps. Water softeners work by removing the temporary hardness (such as carbonates) by replacing it with permanent hardness such as chlorides. This increased level of chloride is unnatural to any environment where the fish or shrimp may be found. While the water is now softer, from the fish’s or shrimp’s point of view the water is still chock full of dissolved minerals (chlorides or sodium) and TDS will still be high. The cautious approach is to avoid using water softeners altogether if you are trying to reduce the hardness of your aquarium water. Increasing TDS We have discussed reducing TDS, but how do you increase TDS the right way? Increasing TDS is one of the easiest things to do. In fact, you could do nothing to the tank and TDS will increase over time. You could add salt or sugar to the water and TDS would increase. However, that increase is due to waste from fish, food, etc. and not always a good thing. And neither is adding salt or sugar - Don't do it ! The main minerals/chemicals that you want to use to increase TDS in an Aquarium is Calcium & Magnesium at 4:1 ratio and to a smaller extent other minerals like Potassium and trace elements. There are several off the shelf products that will remineralise low TDS water, increasing it to a suitable level. If you'd like to Do It Yourself, I even have a recipe here ... Summary One of the most vital aspects of keeping soft water shrimp or fish is the significance of TDS - Total Dissolved Solids. The majority of aquarists will put their attention on the pH only for soft water fish or shrimp but completely forget about TDS. A simple $20 piece of equipment will be able to rectify that. The various years of observation has lead to a realisation that low pH actually means low TDS be it for fish or shrimp. Both water parameter readings need to go hand in hand. We cannot ignore one or the other when you are trying to replicate the aquarium’s environment. The effects of shock can be offset by slowing mixing the water. And this can be important between your own tanks too, as TDS is unique to each tank. A TDS meter is an absolutely essential tool in an aquarist’s cabinet. For the shrimp keeper, monitoring TDS is of vital importance. In an environment where the shrimp are constantly using up Calcium to grow their shells, and dissolved solids are constantly changing due to food, nitrogenous waste being produced, and even evaporation of water can cause fluctuations in the level of dissolved solids in the tank water. This constant fluctuation can cause stress in the shrimp. This stress can lead to a reduction in their immune systems, and sometimes eventuate in death. Close monitoring of TDS is required to ensure the shrimps environment is stable. TDS should never fluctuate wildly. Aim for a constant TDS reading in the tank. In doing so, you might find that you will need a lower TDS reading for water changes in order to maintain a target. For example, if your target is 150ppm TDS, then you might need to aim for 110ppm TDS in your change water. Aiming for 150ppm TDS in the change water will result in TDS rising overtime as dissolved solids gets concentrated in the tank. Over time, TDS continually and constantly rises each day. They enter the aquarium via fish food, water conditioners, plant fertilizers, medications, and any substance that treats water in some way. Water evaporation will also cause the dissolved solids already in the tank to be more concentrated. TDS readings can also be used as an indication of when it is time for a water change. If you see TDS rising to the upper limits of your target TDS, then it’s time for a 5-10% water change. If the small 5-10% water change is still not enough to reduce TDS to your ideal target, another water change might be necessary two or three days later. Don’t rush in reducing TDS. Slow is always advisable. TDS readings also come in handy when acclimatising shrimp and fish. We all know how to drip acclimatise shrimps or fish. This process reduces the impact of large fluctuations in differing water parameters. I often hear of people saying “I drip acclimatised my shrimp/fish for 3 hours” or “6 hours”. But how do you know that 3 hours or 6 hours or even 12 hours was enough for that matter? Instead, rather than acclimatising new shrimp or fish by amount of time, we should be monitoring the TDS. Once the TDS reaches the same reading between the tank and the water the new shrimps/fish came in, then you can be sure that GH, KH and pH will all be matching as well. This can take a varying amount of time depending on how fast you add the tank water and how much water is already in the bag containing the new shrimp/fish. Once TDS is matching, then place the bag or container into the tank for a few more minutes to ensure temperature is the same before catching and releasing your new pets into the tank. It can take 6 hours or it can take 16. It doesn’t matter, but I have never lost a fish or shrimp using this TDS monitoring method of acclimatising. JayC SKF Aquatics http://skfaquatics.com/
-
Total Dissolved Solids or TDS for short is an area of water parameter we talk about very often, and is usually one of the first things we ask about when checking water parameters. TDS and why is it important Preamble: Total Dissolved Solids or TDS for short is an area of water parameter we talk about very often, and is usually one of the first things we ask about when checking water parameters. This article will hopefully go into some depth for anyone who might still be new to the hobby, and likewise, might teach the veterans a thing or two that they might not have known about TDS. I have tried to keep the language appropriate to newcomers in mind, so please don’t expect a paper that reads like a scientific thesis. This article is also written from the perspective of a fish and a shrimp keeper, as I am, and draws from my experiences in these areas. You will see frequent mention of killis, Apistos, and shrimps. For the sake of simplicity, we will regard Electrical Conductivity (EC) to be of similar importance and similar definition to TDS. A definition of EC is the measure of the water's ability to "carry" an electrical current and indirectly, a measure of dissolved solids or ions in the water. Whereas a definition of “Total Dissolved Solids (TDS) is the total amount of mobile charged ions, including minerals, salts or metals dissolved in a given volume of water, expressed in units of mg per unit volume of water (mg/L), also referred to as parts per million (ppm). TDS is directly related to the purity of water and the quality of water purification systems and affects everything that consumes, lives in, or uses water, whether organic or inorganic, whether for better or for worse.” – (source: HM Digital) From the perspective of an aquarist, TDS can be defined as: a count of all the dissolved inorganic solids in the water. TDS gives an overview of mineral content in the water. It does not just necessarily provide information on hardness even though it does include the measurement of minerals like calcium and magnesium. Instead, TDS also includes measurements of all the other dissolved minerals in the body of water. So you cannot use TDS to give you an indicator of hardness, that is, how much calcium carbonate is dissolved in the water. GH is at its heart a measure of divalent cations, namely Ca (calcium) and Mg (magnesium); and we know KH is a measure of carbonate concentration. Both GH and KH can influence hardness and TDS levels – ‘an aquarium high in GH & KH can have a high TDS’. However, a fish tank could have a high TDS reading but still have low GH and KH readings. In this situation the aquarium water might be high in one or more of the other dissolved minerals apart from Calcium and Magnesium. Therefore, TDS is a better reflection of the total mineral content than hardness measurements. In conclusion, Total Dissolved Solids consists of dissolved ionic elements, both cations and anions. Whereas, GH only measures two elements, Calcium and Magnesium. Let’s see what those other minerals, that a TDS meter/pen measures, might be. In chemical terms, if a neutral atom loses one or more electrons, it has a net positive charge and is known as a cation (source: Wikipedia). Cations are elements that can be found mainly on the left side of the periodic table (metals) and when it reacts, they usually become positive ions. Cations include ions such as calcium, magnesium, potassium, sodium, barium, iron, copper and zinc. If an atom gains electrons, it has a net negative charge and is known as an anion (source: Wikipedia). Anion elements can be found on the right side of the periodic table which reacts with metals to take electrons to form negative ions called anions. Anions include elements such as chloride, nitrate, iodine, bromide, fluoride, sulphide, chlorate, permanganate, phosphate and sulphate. Because of their electric charges, cations and anions attract each other and readily form ionic compounds, such as salts. All these ions and other inorganic ions are what is included in the measurement of TDS. Occasionally you will also hear of the term Total Suspended Solids (TSS). Therefore Total Suspended Solids refers to solids both suspended and dissolved in water and is directly related to conductance and turbidity (optical determination of water clarity – how cloudy/clear the water is). Dissolved solids (invisible) are therefore the substances that can flow through the filter media, too small to be trapped. And the substances in TSS include undissolved solids (visible), like bits of plant matter, or detritus and therefore includes substances that can be trapped by the filter media. High levels of TSS also have the following impacts: increased levels of TSS obstruct light and therefore reduce photosynthetic absorption in plants. High TSS can gradually decrease the amount of oxygen produced by these plants. Decaying plant matter uses up a lot more oxygen and subsequently reduces the amount of dissolved oxygen available in the water. Unless there is a significant amount of surface agitation (oxygenation). It’s always a good idea to have your filters, be it air driven or canister, break the surface of the water. It will reduce protein scum off the surface and maximise the oxygen exchange. While TSS is not specifically measured in a TDS meter, it’s good to know the difference between TDS and TSS, as well as its influence in the aquarium environment. Measuring Total Dissolved Solids TDS is the measuring of the amount of salts in a solution. For a lot of applications the amount of salt is indicative of the levels of other stuff in a solution. TDS/PPM meters sold for gardening and aquariums figure the amount of salt in Parts Per Million by measuring the Electrical Conductivity of the solution under test. So a PPM/TDS meter is an EC meter that converts the EC value into PPM values. EC is a measure of Electrical Conductivity from two probes 1cm apart. 1 EC is = 1 microsiemens, to convert from EC to siemens multiply by 1E-6. EC can be converted to PPM by multiplying by 500. PPM can be converted to EC by dividing by 500. To convert from siemens to Ohms is s=1/ohms, you can also go the other way and do ohm=1/s for siemens to ohms. Siemens is also known as Mhos, which comes from ohm written backward. The number 500 used to convert between PPM and EC is called the Conversion Factor. Different salts will have different conversion factors because some conduct better or worse than others. NaCl's is 500, this seems to be the most common standard used, and is what was used for the calibration solutions. Though there is a close relationship between TDS and Electrical Conductivity, they are not the same thing. Total Dissolved Solids (TDS) and Electrical Conductivity (EC) are two separate parameters. TDS, in layman's terms, is the combined total of solids dissolved in water. EC is the ability of something to conduct electricity (in this case, water's ability to conduct electricity). The measurement of dissolved solids is expressed in ppm of NaCl (sodium chloride) – TDS can be compared to Electrical Conductivity (EC) and the approximate conversion formula to get TDS(ppm) = 0.64 x EC mS/cm Conductivity measures electrolytes. Aquarists can now measure TDS levels via tests performed using a TDS meter (or TDS pen) in ppm at a relatively cheap price. Alternatively, you could use an EC pen and convert to TDS using the manufacturer’s conversion factor. Picture of a TDS meter or TDS pen TDS meters are usually calibrated using a solution of Sodium chloride NaCl. While Electrical Conductivity meters (EC) are usually calibrated with a solution of Potassium Chloride KCl. How do TDS pens work? Two electrodes with an applied AC voltage are placed in the solution. This creates a current dependent upon the conductive nature of the solution. The meter reads this current and displays in either conductivity (EC) or ppm (TDS). Electronic TDS meters essentially measures the conductivity of water, ie. how well the water conducts electricity. The higher the concentration of ions, results in the higher the conductivity of the water, and thus the higher the TDS level will be. And most of our softwater shrimp and fish don’t like high TDS. Many brands have meters that use a conversion ratio to change EC (conductivity in microsiemens) into TDS (ppm) along with a temperature compensation. It really does not matter too much, which one you choose to use, since they use the standard conversion for tap water of 0.5. For example, an EC measurement of 300 mS is converted to a TDS measurement of 150 ppm (TDS = EC x 0.5). In fact, most (if not all) TDS pens are actually EC meters that convert to TDS automatically saving the user from performing the mathematical step. There are TDS meters that perform a combination of functions (TDS/EC/pH/temp) which allow conversions to be adjusted between 0.47 and 0.85. There is one weakness with TDS measurements however, it does not measure which ions are responsible for the conductivity. So if you are testing tap water you don’t know if it’s the “good” ions like Calcium, Magnesium, Potassium or the undesirable ions like Iron, Copper, Nitrates, or any other number of dissolved solids that makes up the abundance of the reading. That is why many experienced aquarist will recommend using RO water and remineralising it so you know exactly what is in the water. A few general observations on TDS When water reaches a TDS count of 50ppm it becomes electrically conducting, that is, it’s able to conduct electricity at this level. The EPA Secondary Regulations advise a maximum contamination level (MCL) of 500mg/litre (500 parts per million (ppm)) for TDS. Numerous water supplies exceed this level. When TDS levels exceed 1000mg/L it is generally considered unfit for human consumption. A high level of TDS is an indicator of potential concerns, and warrants further investigation. Most often, high levels of TDS are caused by the presence of potassium, chlorides and sodium. These ions have little or no short-term effects, but toxic ions (lead arsenic, cadmium, nitrate and others) may also be dissolved in the water. Higher levels can be a likely cause for corrosion in plumbing. The visual quality of water is also impacted at levels higher than this. A general observation of high TDS water is that it is slightly alkaline in pH, that is it is lacking in hydrogen molecules. As with everything in nature that tries to reach equilibrium, high TDS or alkaline water will want to seek out hydrogen molecules to reach a neutral state. As such, slightly alkaline water often causes dehydration at a cellular level. Low TDS water on the other hand is loaded with positively charged hydrogen molecules and is therefore slightly acidic in pH. Low TDS water is therefore very hydrating at a cellular level. TDS and Low pH fish When we discuss soft water fish or shrimp that like low pH, what that generally means is that these fish are really low TDS fish. While it is not impossible for many of these low TDS fish to adapt to harder water, and higher pH (and often relatively higher TDS levels), the problem is, especially for South American soft water fish and Caridina shrimp, that calcium and magnesium rich water makes the egg’s membrane harder, and dramatically reduces the chances of hatching. And in more recent experiences, I’ve had greater success hatching killi fish eggs in lower TDS levels than at higher TDS levels. I have also observed that high TDS levels (due to high levels of chlorides, calcium and magnesium, fluoride, sulphides as found in tap water) is generally the cause. This is where it can be a common mistake for many people, including myself, to try all sorts of methods to lower pH and hardness but give no attention to TDS values. This is where Reverse Osmosis water can help with this predicament. I now use RO water to successfully lower calcium and magnesium levels, as well as TDS. This in turn has an impact on reducing pH as well as KH and GH. The fish seemed to be much more contented using this method of preparing clean, low TDS water. And as a reward, the fish promptly rewarded its keeper with eggs which hatched into fry (apistos, rams, other South American dwarfs and Killies). If you want to also match the tank water with the shrimp’s or fish's natural habitat parameters, keep the TDS levels low. What is low? Soft water is generally considered to be in the range of TDS 70 – 150ppm. My personal observations have shown me that high levels of dissolved mineral content in the water, are the main reasons for the failed breeding of many Killis, Shrimp (Caridina) and South American dwarf cichlids. This is attributed to inappropriate levels of calcium and magnesium, and subsequently carbonates and bicarbonates. Placing the emphasis solely on pH alone does not rectify the issue since it can be said that pH is a symptom of the overall mineral content in the water, rather than the actual root cause. To make matters worse, pH down chemicals only adds to the TDS count, not decrease it. When breeding low pH fish, keep your emphasis on TDS instead of just pH. Some aquarists can often find themselves in a situation with tap water parameters that are no where near their shrimp’s or fish's preferred water conditions. My tap water in Sydney for example, is pH 7.8+, and GH & KH are also somewhat high for breeding caridina shrimp, Killi, or Apisto dwarf cichlids, which means we need to find a way of reducing it. We need to really stop thinking about just the permanent hardness of pH, KH and GH, or even temporary hardness for that matter. Concentrate on reducing calcium and magnesium hardness instead. Mixing the tap water with just plain RO at a ratio of 50:50 can be the simple solution to solving the problem of TDS, pH and Hardness. We want to keep calcium and magnesium hardness in check since this can affect the fertilisation of the egg, as the egg’s membrane can get too hard to a point of making fertilisation extremely hard (pun) and nearly impossible. Sometimes I will mix RO water with peat treated water along with tap water to make water whose parameters closely approximates the needs of the Killi or South American dwarf cichlids. Tap water can be included in order to stabilise KH levels and thus keep the pH from fluctuating. Occasionally, I will add my own remineralising DIY mix to RO water to bring TDS up to a certain specific level on the occasions that adding tap water was undesirable, especially for my shrimp tanks. TDS readings for my Caridina shrimp are around 140-150, with a lower KH value of 0-1 and GH of around 5-7 have been proving to be successful for me. Neocaridina dwarf shrimps are capable of tolerating slightly higher TDS levels of up to 200. They might survive higher, but it would be unethical of me to advise you that it’s okay beyond 200. TDS readings for Killi and South American cichlids of between 70 and 110ppm with a stable KH reading between 3 and 7. New soft water low pH fish and shrimp don’t merely survive in this treated water, but instead will thrive and multiply. You will find quite frequently that you will need to mix your water changes to a much lower TDS value that your target in order to maintain the tank’s overall TDS. This is normal, as the dissolved solids in the tank is continually increasing from various sources, like fish/shrimp waste, minerals introduced in food, water evaporation, or even decomposition of plants and organic matter. You might even get to a point where you need to change 80%+ of your tank water just to reset the TDS values. Don’t forget to re-acclimatise the shrimp back into this new water. TDS: Water Changes Many professional fish breeders practice the following method of TDS monitoring; it is one important parameter used to keep healthy fish. You could also use TDS levels as a means of deciding on the frequency of when it is time for a water change. A rise in TDS levels means it is time for some water to be changed, returning TDS levels to a lower count. Sharply increasing TDS levels can also indicate overfeeding, an over-stocked tank, or too much added minerals or fertilisers. But I would use caution in relying solely on TDS readings for water change indications. This is best reserved for those that are very familiar with their tank and understands what the TDS reading is showing. “pH Shock” - Moving fish from one tank to another For many years as a fish keeper, and now also as a shrimp keeper, I have understood changing the pH on fish or shrimp too quickly is a bad thing. It was only until I was researching the importance of TDS, a revelation has come to mind. TDS levels can represent different states of osmosis. Many aquarist have largely believed fish that succumb to what we call 'pH shock' is caused by the rapid variation in TDS levels. This places osmotic pressure stresses on the fish's osmoregulatory mechanisms which cannot become accustomed fast enough to the changing environment and hence the fish goes into a state of suffocation and in many cases can cause death. Fish have been shown to withstand fairly significant pH shifts when the TDS was low in both waters. It was not 'pH shock' as it is often alleged– that is, where the difference in pH is significant between one tank to another. But it was TDS shock! Maybe it’s because TDS meters are not as readily available, whereas, pH kits can be found in every fish shop. So the misguided recommendation was to test for pH, rather than TDS – who knows. One could declare that TDS measurements help to give an indication of the differences in osmotic levels between the water of one tank and another. In water with less total dissolved minerals compared to the amount of dissolved minerals in the tissue of the shrimp/fish, will cause the shrimp/fish to lose fluid from its cells via its gills (over hydration). In high TDS water, it has the opposite effect, they become dehydrated. Which causes the fish to have difficulty passing toxins out via its kidneys. This is a longer term impact to the fish, and you might not notice any impact immediately. IMHO, TDS meters are often the most under estimated tool that can be used to give a good indication of how successfully a shrimp or fish will adapt to the water in one tank to another. As a second reference, reading one of J. J. Scheel’s articles on dissolved solids also brought me to this realisation of ‘TDS shock’. Between 1959 and 1965 Col. Jorgen J. Scheel of Denmark sent out some letters about the science and systematics of killifish to any hobbyist that was interested. Scheel had what might be considered today some unorthodox opinions regarding water chemistry. He felt differences in salinity, or total dissolves solids mattered much more than pH (which could be safely ignored). Given this observation was made over many decades of working with killifish, it's a pervasive argument. Here are the relevant passages from Rivulins [killifish] of The Old World: Page 25 Page 26 TDS can also significantly impact the osmoregulation of the gills. Low TDS can cause the red blood cells to be depleted of water in fish that might not be acclimatised to the low TDS. While in high TDS, the red blood cells in the gills can be saturated with water causing the red blood cells to expand. Both will cause respiratory problems. As a result, always drip acclimatise new shrimp or fish to your tank prior to introducing them. Use your TDS meter/pen to match TDS values in your tank and the water of the new shrimp/fish. It usually takes doubling the amount of water from the tank to match the TDS in the bag of the new fish/shrimp. More caution needs to be placed on reducing TDS levels, compared to increasing TDS levels, as the former seems to be more lethal. Methods of lowering TDS There are several methods of lowering TDS, however, we will focus on only two methods as the other methods are unsustainable in the long term. These sources of low TDS water will need to be remineralised with Calcium & Magnesium mix in a ratio of 4:1. Remineralising raises the low TDS water back to a more suitable amount specific to the requirements of your fish or shrimp. Do not use low TDS water except to top up water loss due to evaporation. RO (Reverse Osmosis) Water Reverse osmosis works by forcing water under great pressure against a semi-permeable membrane that allows water molecules to pass through while excluding most contaminants. RO is the most thorough method of large-scale water purification available. There are a huge number and variety of RO systems around. Studies have revealed how the concept of osmotic pressure can assist in decontaminating water. With a fine particulate membrane and the act of forcing water through that membrane with sufficient pressure, will produce clean water on the other side of the membrane. The clean water is stored and the filtered waste is either thrown away or used for other non consumption uses like watering plants. RO systems can removed up to 98% of all ionic and organic impurities like pollutants, sediment, bacteria and contaminants. And as a result, TDS levels are drastically reduced. The RO filter membranes do not last forever unfortunately. As the TDS of the output water rises, it is generally an indication that the membranes need to be changed. The frequency of use and the level of TDS of your source (tap) water will determine the frequency of replacing the membrane. Deionisation (DI) In large scale DI systems water is passed between a positive electrode and a negative electrode. Ion selective membranes allow the positive ions to separate from the water toward the negative electrode and the negative ions toward the positive electrode. High purity de-ionized water results. Deionization is an on-demand process supplying purified water when needed. This is important because water at this extreme purity level degrades quickly. The nuclear grade deionization resin or polishing mixed bed resin removes almost all the inorganic contaminants in the water increasing the resistivity of the water to a maximum of 18.2 megohm-cm. However, deionization alone does not remove all types of contaminants like dissolved organic chemicals. Deionization filters are not physical filters with a pore size and cannot remove bacteria or particulates. The water is usually passed through a reverse osmosis unit first to further remove non-ionic organic contaminants. RO vs DI: RO purity is relatively continuous while DI gets progressively worse as the resin nears its regeneration point. DI chemicals are expensive and therefore operating costs are higher than RO per litre of purified water. RO membranes are a physical barrier that remove bacteria, viruses, algae and suspended solids, while DI systems cannot remove these contaminants. DI uses two hazardous chemicals, hydrochloric acid (HCl) and caustic soda (NaOH) for regeneration of the resin beds. These chemicals needs special storage and disposal requirements. As you can see, DI water is also uneconomical for aquatic hobbyists. More portable DI systems nowadays use Ion exchange resins to exchange non desirable cations & anions; and replaces them with hydrogen and hydroxyl, respectively, forming pure water (H20), which is not an ion. One type of resin will remove positive IONS, while another type of resin will remove negative IONS. Cations Anions Removed by Cation Resins Removed by Anion Resins Calcium (Ca++) Chlorides (Cl-) Magnesium (Mg++) Sulfates (SO4=) Iron (Fe+++) Nitrates (NO3=) Manganese (Mn++) Carbonates (CO3=) Sodium (Na+) Silica (SiO2-) Hydrogen (H+) Hydroxyl (OH-) (Table care of Puretecwater) You might come across the term "Mixed bed" or "Dual Bed" system - this is a DI filter with both Cation and Anion resins. RO/DI portable systems Modern portable RO/DI systems solve both the individuall RO and DI shortcomings. These systems combine an RO membrane with DI resins to produce near 0 TDS water. The RO removes the organic waste like bacteria, viruses and algae that the DI cannot. While the DI removes the minerals like Calcium, Magnesium, Chlorides, Sulfates, etc that the RO membrane misses. By combining the two, we get the best of both worlds. Most Reverse Osmosis filters you can buy today, like those sold by FSA, https://www.filtersystemsaustralia.com.au/store/index.php/reverse-osmosis-water-filter/aquarium-systems.html are in fact RO/DI systems. Rain water What can be better than water from mother nature? After all, our river systems are made up of water that falls as rain. So this has to be the best source of water, is it not? In most cases it is. However, many of us live in polluted cities, and we collect and store rain water in manmade receptacles that might add to the contamination of rain water. So some form of caution is necessary. If you are confident that the water is collected off relatively clean, rust free roofs and stored in plastic drums, then rain water is a perfect free source of low TDS water. Rain can be sporadic and unpredictable in some countries, so an RO system as a backup is always a good idea. There are other methods of lowering TDS, as mentioned in the next section, but I will not focus on them as it’s not really a preference. I mention it here only as a last resort. Peat All over the internet and on forums, many can attest to using peat in helping to lower pH, GH, KH, and TDS. This greatly depends on your own water conditions and how much the peat treated water affects TDS. If your tap water is particularly hard, you might need more peat to lower the mentioned parameters compared with someone else’s tap water. It is not uncommon to mix the peat with RO water (and/or maybe some tapwater) in an attempt for one to achieve a stable chemistry that agrees with the shrimp/fish you are keeping. The addition of tannins, phenols, humic acids along with the combination of peat treated water allows you to create water conditions close to your livestock’s natural environment. Peat water (even small additions) is positively regarded by many aquarists, as essential for low TDS fish, especially dwarfs such as Discus, Tetras, Corys, Angels, Rams and Apistos. The problem with recommending peat is finding it in Australia is difficult. Especially peat that does not also have fertilisers included. Then there is the extra effort in making peat water, and the need to make it several days ahead of use and store it in containers. The colour that results from the added tannins from peat is also not to everyone’s liking. Distillation Distillation involves boiling the water to produce water vapour. The water vapour then rises to a cooled surface where it can condense back into a liquid and be collected. Because the dissolved solids are not normally vaporized, they remain in the boiling solution. However, some impurities with the same boiling point of that of water can be transferred to the collection water, and for this reason, Reverse Osmosis can produce purer water. The absolute advantage of the distilled water is the complete absence of harmful substances like bacteria, viruses or algae. Considerable amount of cost is required to produce and maintain the thermal requirements for a distillation process. As a result this method is uneconomical for aquatic hobbyists. A quick word on Water softeners Water softeners do not necessarily produce water that is suitable for Softwater fish and shrimps. Water softeners work by removing the temporary hardness (such as carbonates) by replacing it with permanent hardness such as chlorides. This increased level of chloride is unnatural to any environment where the fish or shrimp may be found. While the water is now softer, from the fish’s or shrimp’s point of view the water is still chock full of dissolved minerals (chlorides or sodium) and TDS will still be high. The cautious approach is to avoid using water softeners altogether if you are trying to reduce the hardness of your aquarium water. Increasing TDS We have discussed reducing TDS, but how do you increase TDS the right way? Increasing TDS is one of the easiest things to do. In fact, you could do nothing to the tank and TDS will increase over time. You could add salt or sugar to the water and TDS would increase. However, that increase is due to waste from fish, food, etc. and not always a good thing. And neither is adding salt or sugar - Don't do it ! The main minerals/chemicals that you want to use to increase TDS in an Aquarium is Calcium & Magnesium at 4:1 ratio and to a smaller extent other minerals like Potassium and trace elements. There are several off the shelf products that will remineralise low TDS water, increasing it to a suitable level. If you'd like to Do It Yourself, I even have a recipe here ... Summary One of the most vital aspects of keeping soft water shrimp or fish is the significance of TDS - Total Dissolved Solids. The majority of aquarists will put their attention on the pH only for soft water fish or shrimp but completely forget about TDS. A simple $20 piece of equipment will be able to rectify that. The various years of observation has lead to a realisation that low pH actually means low TDS be it for fish or shrimp. Both water parameter readings need to go hand in hand. We cannot ignore one or the other when you are trying to replicate the aquarium’s environment. The effects of shock can be offset by slowing mixing the water. And this can be important between your own tanks too, as TDS is unique to each tank. A TDS meter is an absolutely essential tool in an aquarist’s cabinet. For the shrimp keeper, monitoring TDS is of vital importance. In an environment where the shrimp are constantly using up Calcium to grow their shells, and dissolved solids are constantly changing due to food, nitrogenous waste being produced, and even evaporation of water can cause fluctuations in the level of dissolved solids in the tank water. This constant fluctuation can cause stress in the shrimp. This stress can lead to a reduction in their immune systems, and sometimes eventuate in death. Close monitoring of TDS is required to ensure the shrimps environment is stable. TDS should never fluctuate wildly. Aim for a constant TDS reading in the tank. In doing so, you might find that you will need a lower TDS reading for water changes in order to maintain a target. For example, if your target is 150ppm TDS, then you might need to aim for 110ppm TDS in your change water. Aiming for 150ppm TDS in the change water will result in TDS rising overtime as dissolved solids gets concentrated in the tank. Over time, TDS continually and constantly rises each day. They enter the aquarium via fish food, water conditioners, plant fertilizers, medications, and any substance that treats water in some way. Water evaporation will also cause the dissolved solids already in the tank to be more concentrated. TDS readings can also be used as an indication of when it is time for a water change. If you see TDS rising to the upper limits of your target TDS, then it’s time for a 5-10% water change. If the small 5-10% water change is still not enough to reduce TDS to your ideal target, another water change might be necessary two or three days later. Don’t rush in reducing TDS. Slow is always advisable. TDS readings also come in handy when acclimatising shrimp and fish. We all know how to drip acclimatise shrimps or fish. This process reduces the impact of large fluctuations in differing water parameters. I often hear of people saying “I drip acclimatised my shrimp/fish for 3 hours” or “6 hours”. But how do you know that 3 hours or 6 hours or even 12 hours was enough for that matter? Instead, rather than acclimatising new shrimp or fish by amount of time, we should be monitoring the TDS. Once the TDS reaches the same reading between the tank and the water the new shrimps/fish came in, then you can be sure that GH, KH and pH will all be matching as well. This can take a varying amount of time depending on how fast you add the tank water and how much water is already in the bag containing the new shrimp/fish. Once TDS is matching, then place the bag or container into the tank for a few more minutes to ensure temperature is the same before catching and releasing your new pets into the tank. It can take 6 hours or it can take 16. It doesn’t matter, but I have never lost a fish or shrimp using this TDS monitoring method of acclimatising. JayC SKF Aquatics http://skfaquatics.com/ View full article
-
I have had a 20 gallon RCS tank for 3 years now. I have moss balls and some JAVA moss, drift wood, and mineral rocks. Only RCS in the tank and some soft shell snails I have in there I breed for my fresh water puffer fish in another tank,. My issue is that the shrimp live 1-2 years and they breed just fine but I never see babies. I see plenty of eggs but never babies. (Well 1 or 2 a month maybe but they disappear) The adults keep going just fine until they die off of old age or something else. I keep about 100 shrimp in the tank and have to replenish 50 every 6 months about. Water Temp I keep about neutral. PH- 7- 7.2 I use distilled water or sometimes Reverse Osmosis water because our tap water goes through a water softener which puts a LOT of salt in it which makes TDS very high so I try not to use tap water at all. I feed every 2 days with Azoo or other good food and not much. Its all gone by the time I feed again. I do add some powder food for the babies and a shrimp bacteria powder (just started that about 6 months ago but has not helped or hurt) Someone told me due to the distilled water my issue is LOW TDS. So I bought TWO different meters. I tested the distilled water and its about zero which is correct. I then tested my water and its about 700. I do top offs and just did a 20% water change last weekend with distilled water. So is that the issue? Should I pull out the mineral rocks? I am thinking tonight to take about 8 gallons of water and drain the tank down about 40% and then fill with the distilled water. I can repeat the process in a week and once I get the TDS under 150 I can add a little shrimp mineral to get it to the 200 range. The reason I am saying that is the TDS could be bad TDS and not the minerals they needs. I ordered some Salty Shrimp Mineral which I heard was good. Should I just give up? Jeff
- 10 replies
-
- 1
-

-
- red cherry shrimp
- tds
-
(and 1 more)
Tagged with:
-
I am new to keeping shrimp and was hoping someone can help me sort through some issue with the water parameters of my tank. The tank is a 4.6 gallon cube, that runs between 72 - 74 (22.22 - 23.33) degrees, 0 ammonia, 0 nitrates and 0 nitrites. It has been cycled and running since December 2015 but about a month back converted to a shrimp tank (removed the fish, did a 100% water change and added sponge over filter). Current inhabitants are a nerite snail and 1 RCS. I initially had 3 RCS, but lost two. I believe this was due to them trying to molt as they both had white lines on their back, but were fine the day before (one after 7 days and another after 12 days). I also have some java jerns, java moss, dwarf sags, crypt wendetti, and some floaters. Current water parameters: TDS: 166ppm GH: 1.7 KH: 0 My PH is also low (6 something if I recall but can measure this again if needed). I am using tap water that I add prime to that is then place in a rubbermaid with a powerhead until I need to do top offs / water changes. So far all I have done is top off on the shrimp tank. My plan is to pick up some Salty Shrimp and add that to the tap water to raise GH and KH. I believe I should be shooting for a GH between 6-8 and KH of 2-5, with a TDS between 80 and 200. I also plan to add some iodine twice a week to try and address the molting issue. Does this seem reasonable? Any additional changes I should be making or consider? Once the water parameters are in order, I would like to add some additional RCS to the tank.
- 1 reply
-
- water parameters
- tds
-
(and 2 more)
Tagged with:
-

How do I correct water parameters please.
puddlejumper388 posted a topic in General Questions/Discussions
Hi everyone, I have spent some time searching (unsuccessfully!) for any threads set up to address how to naturally and chemically treat the more important water parameters. Obviously I'm not talking about temp, but the PH, TDS, KH and GH levels are the ones I'm most interested in. Now I'm country based so the only water I've got access to is R/W and bore (perfectly drinkable from the pump itself, no brackishness) which I have used for 4 of my 6 tanks (tropical and a few lower grade cherries). But I want to better hone in the water condition as best I can so any tricks to raise lower the above parameters naturally or if need be chemically. Or if anyone knows/finds a link, anything will be appreciated. Had put this in the pinned "Shrimps 101" but will try to delete it as it's probably better as a separate post. Being rural makes water choice difficult and some of the values I've tested are way out, hence why I'm seeking advice. Cheers all. -
Hey SKF peoples, I'm just mixing up my RO water with a combination of GH+ and GH/KH+ to keep tiger shrimps in. And I thought I'd share my experience, I gradually added the minerals and measured pH along the way and I thought I'd share the results. I note that the pH may change overnight after letting stand but I have been running a pump in the water to mix it well and aerate it so I doubt there will actually be any measurable shift. As you can see by the results, the GH/KH+ pushed up the pH a LOT! Does anyone else have this experience? I have achieved my desired water parameters in terms of ppm and GH/KH however the pH is unreal.. and this is not the first time this has happened to me either. However t is the first time I have taken the effort to document the fact. I'm planning on experimenting with adding a very shallow layer of the cal aqua labs black earth premium and monitoring the pH over the course of days.. expecting it to slowly drop... Any input is 100% welcome! love n peace will PS the initial drop in pH after adding the first lot of GH+ I understand can be explained (as I have read elsewhere) that when attempting to measure the pH of RO water using a pH meter the device can not accurately produce any result due to the lack of ions/conductivity in the water. 27/05/2016 EC meter HM TDS-3 pH meter pH APIkit KH GH At time of water mixing EC0 ppm0 fresh RO 6.6 after adding 50ppm GH+ 6.3 after adding 30ppm GH/KH 7 after adding 25ppm GH/KH 7.5 after adding 45ppm GH/KH EC300 8.3 after adding 17ppm GH/KH EC333 ppm175 8.3 7.8 3 8
-
Hi all. I am new to this hobby and have been keeping cherries and crs for a few months now. Each species have their own tank, from 20L to 35L. They are breeding well so far and I am going to move them into 55L tanks. The new tanks were added with RO and old aquarium water, which read TDS of 120-150ppm, minimally planted, 240-litre rated sponge filter, black earth premium substrate, temperature maintain about 24-26 degree Celcius. Tank cycle was surprisingly quick with another matured external filter, almost no ammonia till date, I am guessing BEP doesn't leach ammonia like other subtrates. On day 3 after setting up, I put one feeder goldfish in each tank to ensure the water is right. After another 3 days, TDS now reads 230ppm, other parameters stay the same. The goldfish were fed only once with 2-3 pellets. I do add liquid CO2 every other day. I am trying to keep the TDS down to 150ppm for cars, they never breed for me anywhere above 180ppm. My questions are, Why TDS rose by almost 100ppm within 3 days? In terms of breeding, would cherries do fine in 200-250ppm? Managing the parameters is easy in smaller tanks but I am not ready to move them until I can manage the new tanks parameters.
-
hey, just wondering what kind of TDS increases people find in their shrimp tanks on a weekly or monthly basis or whatever? i'm just trying to get a general feel for things, any input will be greatly appreciated :-) if you could also share i think it would be helpful to also know what size tank, how heavily stocked it is and feeding frequency.. thanks in advance, love n peace will